In late September my PhD student Bill Ludt and I traveled to
the beautiful island of Tahiti to attend the 10th Indo Pacific Fish
Conference. This meeting takes place every four years and I have been
anticipating this trip since Bill and I went to the last meeting in Okinawa in
2013. I also knew that I couldn’t go all this way not to collect fishes. As
with other conferences in remote locales, and most field trips, it took a while
to get permits; we were lucky to get them a day before our planned travel began
(even though Bill had been working on them for more than a year).
Also joining us for part of the trip was LSU Biology
professor Brant Faircloth. Brant and I submitted a proposal to run a symposium
on fish systematics focusing on ultraconserved elements. Our session ultimately
became part of a half day symposium called ‘Genes to Genomes: Forging ahead in the study of marine evolution” which we were happy
to help organize. (Special thanks to Dr. Michelle Gaither who was the lead
organizer and did all the heavy lifting.)
Soon after arriving we knew we were in paradise - an
expensive French paradise. My French is passable, but most of the locals we met
also spoke English as well their local Polynesian dialects. I always wanted to
come to Tahiti, not so much for its fishes or the beautiful teal-colored water,
but because I loved the history of Captains Cook and Bligh in this region; and because
of films like Marlon Brando’s Mutiny on
the Bounty.
We went to the central fish market in Papeete around 5am the
first few mornings to see what we could get. We made nice collections of local
wrasses, goatfish, and unicornfish among other colorful, if odd-looking,
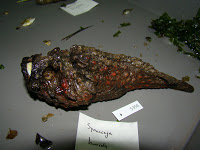
species. At the local grocery store we did come across a large specimen of an
Opah, or “Moonfish” which gained some notoriety recently as being “warm
blooded” – although some ichthyologists remain unconvinced. Sadly the specimen
was too big to collect, and already had it’s gills removed.
 |
| Opah at market (left), butterflyfish (top), and unicornfish. |
We also traveled to the island of Moorea, which is about a
45min ferry ride from Tahiti. This island is home to, among other things, the
Gump Research Station run by UC Berkeley. The Gump helped us get our permits
but we were unfortunately unable to collect on Moorea. We had to settle for a
lovely day snorkeling in crystal clear water surrounded by lush green
mountains.
The conference started a few days after our arrival, and it
had about 500 attendees from around the world. Bill, Brant and I all spoke in
the first session of the first day after the plenaries. The Indo Pacific Fish
Conference is one of my favorites because I get to see many of the European,
Asian and African colleagues I often don’t see at conferences in North or South
America. Bill and I started several important collaborations that hopefully
will make for some fruitful publications over the next few months and years.
 |
| Bill, Brant and I in Cook Bay, Moorea; Bill on stage presenting his talk. |
Although we didn’t hit the markets again during the meeting,
I did get to collect some introduced guppies. The extent of my freshwater fieldwork
was putting a bag down into a sewer off the main road in Tahiti and letting it
fill with water then pulling the bag out of the water to find that 50 individual
guppies had swam into the bag. Many of these specimens were mailed off to a
colleague studying the introduction of guppies around the world. He was very
happy to get individuals from this distant and isolated population.
I’ll spare
you more details about the fish conference and swimming with humpbacks (as I
did) and tiger sharks (as Bill did) and such, but rest assured this was no
vacation (although it obviously wasn’t all work either). The conference was a
great opportunity to talk about our work, including one collaboration that
recently yielded the cover of Systematic Biology (Chakrabarty & Faircloth
et al. 2017; left). That publication created some great opportunities to work with
other scientists interested in using genomic fragments like ultraconserved
elements in their phylogenetic studies of fishes.